Biology's Biggest Mystery: The Origin of Life
Journey back 3.7 billion years to the young earth, as we try to find out how life first began. Was it in a soup of colliding chemistry, a deep-sea hydrothermal vent, or did life rain down on the earth from the cosmos? Plus, the microbial meal that changed the world.
In this episode

Fossil hunting!
with Jamie Jordan, Fossils galore
What are the secrets lying beneath our feet? Georgia Mills went fossil hunting with palaeontologist Jamie Jordan...
Jamie - This is the monster bed that has been excavated out of the main quarry and placed in this safe area for us to hunt through today.
Georgia - Kings Dyke nature reserve in Peterborough. I’m with Jamie Jordan from Fossils Galore and we’ve gone fossil hunting…
Jamie - You never know, you might come across some bones, or teeth, or other prehistoric creatures as well that lived in this shallow tropical sea from the Jurassic.
Georgia - I’m so excited - might I find a dinosaur?
Jamie - You might do. Yeah, you never know. I’ll just get some tools out…
Georgia - Oh yes. Show me your tool set
Jamie - There’s a hammer there. Chisel as well… you got it?
Georgia - Hammer and chisel.
Jamies - We’ll start heading down and I’ll show you how to find them... We’ll be looking for material like this one over here. Can you see how shelly it is?
Georgia - Yeah.
Jamie - What we’ve got here is we’ve got lots of ammonites which are prehistoric cephalopods. They’d have lots of tentacles - a little bit like the modern day nautilus that are swimming around today. As you can see there are hundreds of them just everywhere. Like I was saying, every centimetre of clay is 10,000 years of time. So let’s find you a rock which you can split open… you’ll need a hammer.
Georgia - Ready… [hammer sounds ]. What have we got in here?
Jamie - You’ve got lots more ammonites in there.
Georgia - This is an ammonite hotbed.
Jamie - And you’ve also got a fish poo in there as well
Georgia - Oh, fish bone?
Jamie - No, not quite. Fish poo - also knows as coprolite
Georgia - That’s the classy term for it.

03:26 - What was Earth like when life began?
What was Earth like when life began?
with Professor David Rothery, Open University
Back when the first life got going, what was earth actually like? Georgia Mills spoke to David Rothery from the Open University..
David - We’re pretty sure that the Earth and the other rocky planets in the solar system formed about just over 4½ billion years ago through a series of collisions between progressively larger and larger clumps of debris called planetary embryos.
The early Earth was very different from the Earth we see today. After the last large collision, which is probably the collision, which formed our Moon as debris round the Earth, you were left with a body which was, essentially, molten on the outside and iron core in the middle. The outer part would have been molten and it slowly froze over from the top down giving you an early crust. That would be unstable, it would be rifted apart many times, and you’d have eruptions onto the surface. So the surface would be continually reforming itself and the atmosphere above this surface would not be breathable anyway. There was no free oxygen; there was a lot of carbon dioxide. There was nitrogen which we still have today but, apart from that, carbon dioxide and sulphur dioxide - it would have been unbreathable. Hot, unstable and no breathable atmosphere.
We can trace it back to nearly 3.8 billion years old. Beyond that the record is obscured because the Earth was being hit still by the kind of debris which formed the large basins on the Moon. There was still debris around in the solar system so the Earth would have been hit periodically by impacts which would sterilize a large part of the surface. It’s possible that life started more than once but was then obliterated and had to start over again. So we could have had life older than 4 billion years ago but no traces of that survive.
Georgia - We were back in time when we know the oldest life was starting to appear - 3.8 billion years ago. What would the Earth have been like and how do we know?
David - The geological record is pretty patchy but it’s fairly clear that the continents had knot grown to anything like their present size by then. There would only be small continental areas rather like present day islands arcs. If you think of the island arcs in the Pacific, or think of Japan or bits of Indonesia, if you will, but no large continents. That continental crust hadn’t grown and above this an atmosphere which was as dense, possibly denser than today, but no free oxygen only carbon dioxide, nitrogen, and sulphur dioxide. It’s not until 2 billion years ago that the oxygen levels started to creep up because that’s when photosynthetic organisms appeared in the sea that would start breaking carbon dioxide apart and liberating oxygen.
Georgia - So when we look at the geological record, how can we tell there wasn’t oxygen around or there was this sulphur and this nitrogen?
David - You can tell the lack of oxygen from the minerals that form. Today many metals, for example, in the surface environment will oxidise - iron will turn to rust. You get none of this going on 2 or 3 billion years ago. The oxygen levels really were miniscule at that point. So it’s a very difficult jigsaw puzzle but fascinating to think what the Earth would be like. You wouldn’t recognise it if you flew by in a spacecraft because it would be a very watery looking planet. There’d be shallow oceans everywhere and small clusters of islands peeking up above the oceans.
Georgia - Do we know anything about what the temperature was like?
David - Surface temperatures in the past are probably quite variable. The young Sun would have been a little bit fainter than it is today so giving slightly less warmth. But if we had more carbon dioxide in the atmosphere during the past, as we think we did, that’s a good greenhouse gas so it would have trapped the solar heat more efficiently. So fainter Sun but stronger greenhouse gas effect, you might think they would balance out.
But there are traces in the geological record that more than once in the past the Earth was trapped in what’s called an icehouse condition where the surface water everywhere would have been frozen over. It would have been a ball of ice and these ice house conditions lasted for tens of millions of years at at time. So, we’ve got icehouse conditions - a snowball Earth some of the time but mostly exposed water in between.
Georgia - Everything you’ve said to me so far about early Earth is the fact that there’s no oxygen, it’s been very cold, it’s had lots of stinking sulphur everywhere, there’s only rocks to eat, it doesn't sound to me like a good place for life to be?
David - The early Earth would not be a good place for our kind of life, but our environment that we do so well in has been manufactured by life. It was microbes that liberated the oxygen. It was microbial plants and then the higher plants spread to land. Until that happened, our kind of life could not exist at all. The early Earth was a great place for microbial life that doesn’t like oxygen and would find the early Earth a very pleasant place to live, but not for us. If you say was it a good place for life, you have to specify what kind of life are you thinking of?
Georgia - Understanding that life way back then was very different today has implications in our quest to find E.T…
David - If we’re looking for life elsewhere we shouldn’t expect planets to be in the same state that the Earth is. If there is a exoplanet similar to the Earth, we’d hope to be able to identify it in it’s own atmosphere, but the atmosphere’s been put out of balance by living processes, and that’s probably the easiest kind of extraterrestrial life to detect at remote distances. But I would expect that there are many exoplanets which are Earth-like which have not developed yet into the state that the present day Earth is, which still have these primitive atmospheres hardly touched by life. So there could be lots of worlds with microbes living on the deep ocean floors which leave no clear trace in the atmospheres yet so they’ll be hidden from our view, even with our best instruments. So there could be lots of life-bearing planets which are going to be exceedingly hard to recognise as such.
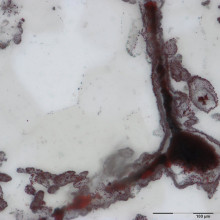
09:53 - The world's oldest fossil?
The world's oldest fossil?
with Dominic Papineau, UCL Earth Sciences and the London Centre for Nanotechnology
Where is the world's oldest fossil, and how can we be sure it's really evidence of life? Georgia Mills spoke to Dominic Papineau from UCL, whose interest in life elsewhere in the Universe is taking him deep into the past...
Dominic - I’m going about it the only way that technologically we currently can, although we do have rovers that are capable on Mars to directly search for signs of life and there’s also the SETI programme. My approach is mostly looking at the very distant past on the early Earth and trying to identify signs of life and understand the environmental conditions where these microbial communities were living because we’re obviously talking about microorganisms. At that time there were no trees, fungus, or other animals or this microscopic life that we see today wasn’t there in most of the Archean probably.
Georgia - So you’re searching for something very, very small - how do you even start?
Dominic - You need to target, I think, the right rocks. You can narrow it down to some of the oldest rock sequences our there and this rock sequence that I’ve been at in 2008 in northern Quebec, the Nuvvuagittuq supercrustal belt, is known to be at least Eoarchean in age since 2004. The Eoarchean is the first time period this sedimentary rock record appears.
Georgia - Was it case of hey, these rocks are really, really old, let’s have a look in them, and then you’re just hoping you might find something?
Dominic - Not quite. The Nuvvuagittuq supracrustal belt is about 3 kilometres in length and the banded iron formation unit that is smack in the middle of this has been known for more than a decade now. I was studying banded iron formations so I was interested in these particular biffs as well because these are very ancient. They’re as old as those I was looking for from Isoa and the island of Akilia in Greenland, and that was going to be another example of eoarchean banded iron formations. So I went there in the hope of finding similar things as what other people had found and I myself had found. Even better I found the red banded iron formations with concretions so ‘eureka.’
Georgia - Is the combination of the fact that they were very old and the type of rock that made them of interest to you?
Dominic - Yes, exactly.
Georgia - When you found something, what did you find - what did it look like?
Dominic - I was there specifically for the banded iron formation so I walked a long strike back and forth. It was striking to me that at one particular site, very localised, there were outcrops of this red banded iron formation. When I was walking on it I was carefully looking at the rock and I could see these rounded spherical structures which I had seen in the Hammersley before. So I knew that these concretions had something, so I sampled them and that’s exactly where we found the microfossils.
Georgia - You’ve got a selection of these rocks in your cabinet in your office. They’re very pretty but I would never expect they contained any kind of life.
Dominic - If you go to the Museum of Natural History, you can see very often that marine animal fossils are preserved in these rounded bits of rock. They’re called concretions; they’re called limestone concretions. They have various names and the reality is that in geology we don’t really have a good explanation for how they formed these particular structures. But we know that in the recent young geological record that they’re associated frequently with animal fossils.
Georgia - Can we go and have a look at your fossils then?
Dominic - Sure… This is what we see.
Georgia - Oh Wow!
Dominic - So that’s what you say - oh wow.
Georgia - So this is a slice of the rock from your office - a very thin slice under a microscope.
Dominic - They’re a little thicker than the usual ones so we’re experimenting with different techniques now. Yes, this is what these things look like.
Georgia - Id’d have a photo of this in my house. It’s a bit like Jackson Pollock or something - a sort of white background and then little flecks of red and black.
Dominic - And there’s a twisting like a spiral.
Georgia - Oh yeah. That’s like a corkscrew.
Georgia - In the slide, amongst the flecks of red you can clearly see a black spiraling tendril. Dominic suggests this could have been made by a microscopic creature, maybe similar to modern day bacteria. But could these shapes simply be caused by geological processes?
Dominic - So far we’ve had one substantial criticism and that is that there are these so-called chemical gardens that can grow tubular structures. Other such non-biological experiments have been successful at growing these filaments that are sometimes twisted so they have morphologies that look like they could potentially be analogues to features that we observe. But if we just consider this kind of comparison based on the morphology, this is one of the main reasons why there’s been so much controversy in the past. It’s because there's very little lines of evidence. But if you consider the bulk of our lines of evidence - we have twelve independent lines of evidence - so I think this is bullet proof.
Georgia - The twelve lines of evidence are the various shapes and chemicals found in the rock, The patterns, the radioactive isotopes, they’ve all been associated with life. Some on their own can appear without the presence of life but, all together, Dominic is confident he has the oldest evidence of life on Earth. Which is pretty exciting...
Dominic - I celebrated with my wife. But when my student, Matt Dodd, took these images and did the due diligence, and he catalogued the images and mapped everything that he was looking at as I instructed him, he brought to me this very compelling images. At some point he brought me this fossil with the twisted stalks and these were very compelling in my opinion. So this was ‘yay- we have the oldest fossils!’. It’s exciting but, ultimately, it’s what it implies with respect to the rapidity that life arises on the planetary surface. The conditions in which it arises has implications for evolutionary biology because many of these microfossils that we find are similar to microorganisms that are living today in these kinds of environments. There’s a continuity in biology that tells us that some organisms haven’t changed much over all this time so it has implications for the origins of life. It has implications for exobiology. I think it’s an important discovery but it’s one that’s going to be followed by many more to come.
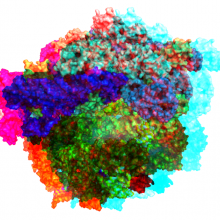
17:44 - The RNA world hypothesis
The RNA world hypothesis
with Professor Laura Landweber, Columbia University
Could RNA be the molecule which started life on earth? Georgia Mills spoke to Columbia University's Laura Landweber.
Laura - Getting some of these raw materials organised would have been a really critical step. And also getting them encapsulated today into what are cellular structures or in what could have been other ways in which compartmentalisation could have captured the raw ingredients that were necessary for life.
There are many steps that would have had to have taken place, but a key one along the steps towards the origin of modern life is how information arose, and how that information could become transferred into functional properties. So we have metabolism in cells and we also have genomes in cells, and linking those two together is one of the most critical steps in life’s origin.
Georgia - Genomes are the sum total of the DNA of a living thing, it’s the instruction manual you need to make proteins, and copies of yourself, and metabolism is the process of obtaining energy from something in the environment.
Laura - Metabolic functions - the notions of another cell’s metabolism, the day to day events that go on in the cell. Today they are primarily catalyzed by proteins.
Georgia - Proteins take on many important functions in our modern day cells. Including our cells’ energy production, and also DNA function.
But here’s the rub. To make proteins today we need the coding instructions from DNA, but DNA – it doesn’t work without the help of proteins. We have a chicken and the egg paradox. How did it first get started?
Laura - There’s a real chicken and egg problem. People have wondered for decades which came first - the information content of a cell, which is today largely in the form of a DNA genome or the functional components of a cell, today largely supplied by proteins.
So, to answer, if we can, this chicken and egg paradox - which came first? The structural properties of proteins that can perform metabolic roles or DNA. Which came first? To answer that question RNA came to the rescue.
Georgia - RNA is very similar to DNA. Just like DNA it’s made from four bases and can form long chains. Today, RNA is used as a kind of middle-man – helping to send messages around. But there’s a clue in one part of our cell as to another role RNA may have had.
Laura - Housed within all of our cells is a big factory called the ribosome. The ribosome takes RNA sequences and makes proteins. One key that we learned from the structure of the ribosome is that at its catalytic core, the functional part of the ribosome that’s really in the business of making proteins, is only RNA, and that helps us understand that actually you can make proteins from RNA. So you can take RNA molecules and add that to what’s called a ribosomal RNA and make something out of it that becomes a protein. There’s a lot of protein itself in the ribosome that helps make other proteins but it would be a bit of an ontological quandary if you needed protein to make protein.
So the fact that you can make protein from RNA helps us build a model for how proteins and protein synthesis evolved from a more primitive system on our planet. It’s beautiful and elegant to recognise that at the catalytic core of the protein synthesis apparatus in pretty much all cells on our planet is an RNA machine, and so that’s called a ribozyme - it’s also part of the ribosome. These factories for producing proteins are comprised of this mixture of RNA and protein that today function together. But it helps us understand how simpler structures built entirely of RNA, probably assisted by smaller bits of proteins, namely the immuno acids themselves, could participate in complex processes.
Georgia - The idea that RNA came first and was helping to build proteins and provide this information is known as the RNA world hypothesis. But how did the RNA itself get created?
Laura - The building blocks of RNA are four letters - A, C, G, and U. The formation of those original building blocks is something which is challenging to explain - some researchers have recently made some terrific progress. But we can also look to our own cosmos to get the basic understanding of where some of this information could have arisen. Even if you look on pieces of meteorites, you can find some of the building blocks for both amino acids and for nucleic acids there.
One of the strengths of this field has been it’s history in organic chemistry, demonstrating the synthesis of the nucleotide adenine, was one of the first experiments, the Oro experiment. It was a long time before another lab demonstrated the synthesis of some of the other nucleotides, but now we have a good understanding that it’s feasible to synthesise them on an early planet. One can even detect the raw precursors to a lot of these nucleic acids in meteoritic material, which means that the precursors for life, as some like to say, would have been raining down on our planet in it’s early days arriving from interstellar dust as well as meteorites, and also from comets. Themselves replete with water, they would have delivered possibly a lot of the opportunity for the raw organic materials needed to create the building blocks of life on an early planet.

25:52 - Early earth simulations
Early earth simulations
with Dr Markus Ralser, Francis Crick Institute
How do we investigate what the early earth chemical reactions might have been? Georgia Mills spoke to Markus Ralser, who is unconvinved by the RNA world hypothesis...
Markus - Historically speaking, many people have tried to explain everything with the emergence of RNA and I think this is very difficult.
Georgia - Markus is unconvinced RNA could really have pushed along those early reactions needed for life - what we call metabolism…
Markus - Every cell consumes a lot of nutrients and this is very essential for life. Therefore cells and systems to convert all of these different nutrients into the molecules that your cells actually need, and that’s what we in biochemistry call metabolism. The way it works is a series of several hundred reactions that happen in all of our cells and from a lot of different angles we think that metabolism had not many origins in evolution, but probably just one. It’s very important to understand those origins of metabolism to get a feeling about which processes enabled life to emerge.
Georgia - So Markus is investigating this from the point of view of those reactions themselves, looking at just how they work…
Markus - We systematically test so we know which reactions are important for metabolism and they are typically catalysed by enzymes. A few years ago we stumbled into some reactions which looked like those enzyme driven reactions but they don’t require enzymes. Then we used analytical instruments called mass spectrometers which allow us to measure thousands and thousands of samples and they systematically test which reactions can happen in those samples.
Georgia - So one of the problems with metabolic pathways is that they need these enzymes, these proteins to help them happen. But what you’ve said is that some of these can happen without the help of proteins, they can just occur anyway. And so you’re throwing together a load of chemistry in an early Earth simulation and then just seeing which of these reactions you can recreate?
Markus - Yes, exactly. For modern life we need enzymes, but the difficulty is that enzymes themselves are made up of products of metabolism. So you end up in a chicken-egg problem and to know what was first. For a long time this was the answer of the question but then people started to see that you can do reactions as they happen in metabolism. Without enzymes, the hypothesis that the whole thing started without enzymes has gotten a huge boost.
Georgia - How do you simulate the early Earth? In my head I’m just imagining a room where you’ve made it look like early Earth but I’m assuming it’s not like that?
Markus - It’s very small volumes of liquids so we are not simulating ten thousands of litres of ocean. We simulate little droplets, 40 microlitres, 50 microlitres, and we add different components that people believe could have existed here on Earth. Those people we talked to, they obtained this information from sediments of stones that they find around the world, and they can date them to this period. Everything is preserved from the time, so a lot of the things one needs to affirm, but there is some solid evidence from geoscientists which molecules made up the early planet.
Then we had the metabolite that we know that are essential for life. We know that they had to play a role at the very early stages of life because now they are essential for life, so that’s our logical view. Then we see reactions can they do without enzymes present in those conditions, and very often we see that many of the reactions that these metabolites undergo are the ones we can find again to be essential for the life of our cells.
Georgia - Do you do anything to this simulation - do you add heat or energy in any way or do you just leave it there and see what happens?
Markus - We did different sorts of things. In the beginning we started with heat because many people think chemical reactions are accelerated by heat and this is typically true. So many reactions require a certain activation energy to happen. Meanwhile, we have expanded this and we test all sorts of different environments. One of them, for instance, is ice. So we see a surprising high reactivity of metabolic reactions to happen in frozen conditions. So there is a huge span and I would say at the moment the answer is open what the right environment was for those processes to start.
Georgia - So you’re putting these metabolites in and you’re putting them under certain conditions, and you’re finding without enzymes they can react and make what exactly?
Markus - They make other metabolites that are part of our cells. This is then not stopping at one metabolite, then one metabolite forms the next metabolite, and the next, and the next, and this results in an entire network. We can then take those networks and compare them with the networks as they happen in our cells. The closer we get, it’s more likely that we have found the origin of such a metabolic system.
Georgia - Do you think then that this metabolic reactions just starting cropping up in this early Earth and, I guess, then what?
Markus - I would say it is the other way round. In order to have life we have to have chemical reactions that happen, and life needs a fundament to build on. When you start to think about how metabolic pathways evolve, you need to have a system that produces a product. Because only if you have something that provides an advantage can the selection procedure start. Now again we have a chicken-egg problem here. If you need multiple enzymes to form a product, but you cannot form the product to select for you have nothing to start with. So we think that these chemical networks were the starting point for evolution to pick out those molecules which provide an advantage and they could select upon. So you can imagine you have a system with ten reactions and then one of them is the slowest or the least efficient reaction, and this is the reaction that evolution can improve first in order to create a product better. If you need to start to work on all ten reactions at the same time, it would be a system which would be much more likely to fail.

Did life come from space?
with Milton Wainwright, The University of Sheffield
Did we originate beyond the stars? Milton Wainright from Sheffield University explained to Georgia Mills why he thinks it's likely...
Milton - Now my view, the one I’m working on, is that life came from space - so-called panspermia. Panspermia means seeds everywhere, so the idea is the cosmos is full of life and it floats around or it arrives in meteorites, and when it hits a planet life is delivered there. So it’s opposite to the general chemical theory which most scientists believe in, the idea that life originated on Earth. We’re saying that life does not necessarily, it could have done, originate on Earth. We think life came in from space, and it continues to come in from space as we speak.
Georgia - Why do you think this is the case?
Milton - One of the problems with the chemical origin of life is that it happened very quickly. Many people think it was too fast for it to have evolved through the chemical theory. We believe that as soon as the Earth was cool enough, imagine these organisms coming in all the time. As soon as the Earth cooled enough, then the organisms could take off, multiply, and so on, and that would be very rapid.
Georgia - How do you go about testing this or investigating this?
Milton - Neither theory can be really proven. The chemical theory - the idea that life came from chemicals. We can keep on playing with different combinations of chemicals but we can't actually prove it. What we’re doing is we’re sending balloons to the stratosphere to see if life is coming in. So the idea, basically, is that if life originated on Earth from panspermia, if it came in, then presumably it will be coming in now because nothing fundamentally has changed. If we catch life coming in, then that’s the possibility that life originated in this way.
Georgia - So you’re sending up these balloons. How do they work and what are you looking for?
Milton - Basically, the balloon goes up to a height of about 30 kilometres. It carries with it kind of a black box. Well, this opens in the stratosphere - we use GPS to locate the height and position and so on. When this drawer opens, whatever is falling falls onto these very small stubs, and these stubs can go straight into a high powered electron microscope. So anything that falls on the stubbs we can look at the surface, and this is how we do the work.
Georgia - How can you be sure what falls on the stubs is from outside of Earth?
Milton - Let me tell you what we found first. We found a number of very unusual organisms. We’ve not found them on Earth and we can use the machine to detect that they are biological. Now we don’t know what they are, so we call them biological entities. The question is, of course, the critics will say there’s plenty of stuff on Earth, there’s plenty of life on Earth; it’s just floating up to this height. I might point out that 30 kilometres is an extreme height.
When we do modeling studies we find that the only particles that could reach this height would be around 5 microns. The organisms we’re finding are 40 microns so they’re much too big, theoretically, to go to that height. The main reason we think they’re coming in is very straightforward. We do not find on our samples any grass, pollen, fungus spores. If this life was coming from Earth our samplers would be covered in this material.
The other main reason is that some of the particles form impact events - that is they form little craters. This means that they’re coming at speed. They’re not kind of lazily floating up from Earth; they’re coming from somewhere at speed. Again this is suggestive that they’re coming in from without.
Georgia - These organisms - have you found DNA in them? Tell me about them - what are they like?
Milton - Let me tell you why we know they’re organisms first of all. They have what we call bilateral symmetry; that means that they’re equal on either side. If this was a bit of fluff then you wouldn’t get this kind of bilateral symmetries. If you think of yourself, if you put a line down the middle of you, you’re equal on either side, so that tells us something; that tells us they’re organised.
The question then is are they biological? The electron microscope that we use is very fancy. We can put a crosshair on our sample, press a button, and look at the elemental composition. We find it’s made of carbon, oxygen, and a little nitrogen. Now that is the exact signature for an organism. So they’ve got form, they’ve got structure, they’re made of biological material, and this is why we think they are living organisms.
As I said before, we can’t correlate them with any known organisms but, of course, you could argue that’s just because we haven’t found them. But they're there, they’re out there and they’re coming in from the stratosphere.
Georgia - What would you need to find, or what would you need to be able to do for people to start accepting that these things are indeed from outside of Earth?
Milton - The first thing people could do was repeat our experiments of course. Science is based on repetition. But the kind of acid tests that we would like to do is what’s called an isotope fractionation. Basically, organisms are made up of carbon with different isotopes, and if an organism came from space it might have a different isotope ratio to the ones on Earth. If we could find the machine that could do this, we could again put a crosshair on our sample, press a button and get the isotope fractionation and that would tell us whether it comes from space or Earth, possibly.
The problem with all these kind of experiments is you predict them, and you think about them, and then some other problem turns up. But that’s a very, very difficult experiment because these organisms are very small, so it’s not easy.
Georgia - If you’re right and these things are from outside of Earth then you have found the first ever evidence of alien life?
Milton - Ha ha yeah, right. The trouble is convincing people and that’s the big trouble. I give talks about this all over the world and people are very sceptical, of course, as rightly they should be. But no-one yet has come up with a reason why our data is wrong. Now we call this neo panspermia - neo for new. And we’re saying, as you walk outside any building, you’re covered with these organisms from space. They’re coming in all the time, they’re coming from the year dot and this is where life, we believe, originated from.
Georgia - So we’re setting everything, searching really hard for aliens, but we’re just covered in them?
Milton - Yeah. Of course they’re extremely small so you wouldn’t see them.

39:23 - Life in a hydrothermal vent
Life in a hydrothermal vent
with Dr Nick Lane, University College London
Could hydrothermal vents be where life originated? Dr Nick Lane told Georgia Mills about the striking similarities between vent pores and cells...
Nick - Essentially, these vents microporous labyrinths of interconnected pores, and the pores have got very thin inorganic walls around them. ‘That’s what the barrier is, the walls around these pores, and the pores may be a few micrometres across, and perhaps just a micron thick. So they’re bigger than cells but we’re getting down to a cell-like size and it has the same structure as cells, that’s what’s intriguing about it. You essentially have a pore with a barrier around it that’s equivalent to the cell membrane and, effectively, a charge on that barrier. So we have a kind of topology which is equivalent to cells.
Georgia - Right. You think this kind of structure, the fact that it’s similar to cells and it’s got this barrier like a cell would have a membrane, means that it’s possible these were where life started?
Nick - That’s my view. You would find lots of people who would sweep that aside.
Georgia - Nick believes that we might be missing something when running simulations of how these first organic molecules were created. And that missing piece of the puzzle, is looking at how cells do it today, which is through using carbon dioxide and hydrogen for energy, powered by an electrical charge on their membranes.
Primitive creatures called methanogens could provide a clue...
Nick - The simplest cells like methanogens, for example, they live from carbon dioxide and hydrogen, so just gases. The kind of gasses that just bubble out of the ground. In fact, hydrogen gas just bubbles out of hydrothermal vents. They don’t react easily, hydrogen and CO2; if they did we could probably fix global warming by stripping CO2 out of the air, and we could fix the energy crisis by making synthetic gasoline. So I’m sure people have been looking at this behind closed doors for a long time.
But this is what methanogens do, and to get them to react they use some of the simplest, most ancient proteins that we know about. Things like ferredoxin, which are iron sulphur proteins and the cofactor that does the chemistry is, in fact, a very small mineral made of iron and sulphur. These are the kind of minerals that form spontaneously in the vents we’re interested in.
The other thing that the methanogens do is they use this electrical charge on the membrane, as well as these iron sulphur proteins to drive the reaction between hydrogen and carbon dioxide. That’s what I think could happen spontaneously in the vents because those natural structures, barriers including these iron sulphide minerals are natural proton gradients that are equivalent to the charge on the membrane. So the prediction is that it ought to drive that reaction and, if it does, then we can look for organic molecules being formed that way.
Georgia - So the hydrothermal vents provides us with a network of pores, similar to cells, a natural charge on the barriers, which could drive reactions, and they’re rich in the chemicals which are used by all cells today to grow. So can we recreate this environment to look for these reactions?
Nick - We would love to do that. The issue, of course, is that these vents can be 60 metres tall. Today, the few that we know about, come in field which can be kilometres square across, and we think 4 billion years ago that they could have spread all across the entire sea floor. So the whole planet becomes a chemistry lab from that point of view. And there’s millions of years to spare, and there’s high pressures, and high concentrations of hydrogen, so to try and do it in the lab is a joke in the sense that we cannot possibly simulate very much of that.
But what we can simulate is the structure. In effect, what you have barriers containing iron sulphur minerals and those are semiconducting, which means electrons can pass across this barrier. One one side we have a simulated an alkaline hydrothermal fluids, the kind of things that we see in the vents today down at the bottom of the oceans.
On the other side, what we think would have been an acidic early ocean, mildly acidic - maybe Ph6 or something - with lots of carbon dioxide in it. The idea is that that difference in Ph (difference in acidity) will help drive that reaction transferring electrons across the barrier. So that’s the kind of experiment that’s relatively easy, conceptually at least, to set up in the lab. The reality is that even very simple systems turn out to be more complex than you can really control.
Georgia - Have you had any results yet?
Nick - It looks as if we are able to make simple organic molecules, and from those simple organic molecules it’s relatively easy to go on and produce more complex ones like, for example, ribose or deoxyribose, which are the sugars used in DNA and RNA. There’s so many steps, that we’re really probing the ones that seem easiest for us to do at any one time rather than the most coherent set of rational steps, we’re doing the ones we can actually do.
So there’s big gaps everywhere and we’re trying to repeat a lot of what we’ve already done. I suppose the normal scepticism is if you can’t repeat everything with different people doing the same experiments then you begin to worry that someone did it wrong, or there was some reason why it’s not believable. You realise when you’re looking for a tiny amounts of trace substances that are actually found on everybody’s breath, for example, you’ve got to be really careful. It’s very easy to make a fool of yourself by thinking you’ve made something when it turns out that you didn’t at all. It as just on someone’s breath.
46:08 - Endosymbiosis: the dinner that changed the world
Endosymbiosis: the dinner that changed the world
with Maureen O'Malley, University of Bordeaux
What is endosymbiosis, and how did it lead to life as we know it? Georgia Mills spoke to Maureen O'Malley...
Maureen - There’s been quite a debate about this and in the late 1960s, Lynn Margulis, very famous for the gaia hypothesis, she put forward this idea that it had been around for 100 years or maybe even longer, that it’s due to endosymbiosis. That one cell swallowed up and retained another cell inside it, and that this cell eventually became the mitochondrion. Once you understand how the mitochondrion gets there, you might understand how you get this kind of intracellular complexity that makes eukaryote cells.
Georgia - So one cell eats another one, and it doesn’t get digested, it just stays there. It’s now thought that before this unusual meal took place the cells were already starting to become more complex on their inside – somehow creating these compartmental barriers.
Maureen - What you imagine is they get a mutation of some kind that generates all kinds of membranes inside the cell, and gives the cell the capacity to move things around in a more sophisticated way. Ultimately, having those membranes inside the cell helps make compartments and so when, eventually, one day this cell gets a bacterium inside it, it holds onto that bacteria with the membrane and eventually converts it to the mitochondrion.
The same things happens, a bit later on (another half billion years later) with what’s called the chloroplast. So this was a free-living, photosynthesising bacterium which gets swallowed up by one of these complexifying cells. And because of these membranes and other structures inside, it’s held there and made to do work.
Georgia - In this acquisition - what are these cells doing? Are they trying to eat them, or is it a mistake and, either way, why doesn’t it get destroyed when they’re inside this cell?
Maureen - First, I’m sure it happens many, many times and by far the majority get destroyed or digested. I think there are different points of view but you can imagine, if you’ve got a membrane that allows you to swallow up other cells, this is a great source of food.
So you’ve got all these other bacteria and archaea out there, you’re a cell that’s getting bigger yourself because you’ve got different membranes and compartments inside, you want food. And if you’re able to swallow up cells, you’re basically taking them into your body, which is just one cell surface, and then if you happen not to digest one or if one of them is something like a parasite even and manages to stay there despite you trying to digest it, but eventually it might happen that the cell that’s ingested is retained.
Once that happens and if it can be passed on to the next generation and, if either it’s not causing harm or it starts to cause a little bit of advantage, that thing will be retained. The exact circumstances are still, I think, quite a mystery.
Georgia - Do we have any evidence? How do we know that cells didn’t just build their own mitochondria? Why do we think there was this external event?
Maureen - This is a great question and I think it’s a very nice historical one too, and this is why Lynn Margulis is so important to the story. People had thought, way back in the 1890s and even before, that perhaps the mitochondrion and especially the chloroplast were bacteria. They looked like bacteria, they had these membranes around them. They in some ways seemed to do metabolic activities that looked like bacteria but that isn’t conclusive evidence.
So what Lynn Margulis did is she gathers together some of the structural evidence. Look at their membranes; what kind of membranes have they got? She also looks at what they’re doing and how they’re doing it. She looks at the structure of various parts of these endosymbionts. What she’s then able to do is draw on the molecular evidence.
Now she doesn’t do any of this molecular stuff, but what happens is in the 70s and 80s, what begins to flourish is the idea of using molecules to understand evolution. So you take molecules from an organism and then, using various evolutionary models, you’re able to extrapolate back to the past. As people look at the DNA from the mitochondria and then DNA from the chloroplast, by the beginning of the 1980s it’s conclusive the DNA of the chloroplast, and the DNA of the mitochondrion is the DNA that’s the most closely related to contemporary bacteria. And because of the other structural things of having membranes, having things inside the mitochondria and chloroplast, everybody is convinced by this. It takes a few years, but what was once a sort of strange, marginal idea even as late as the 60s, becomes a completely standard thing to think by the 80s and 90s.










Comments
Add a comment